Toothed Top Shell Snail
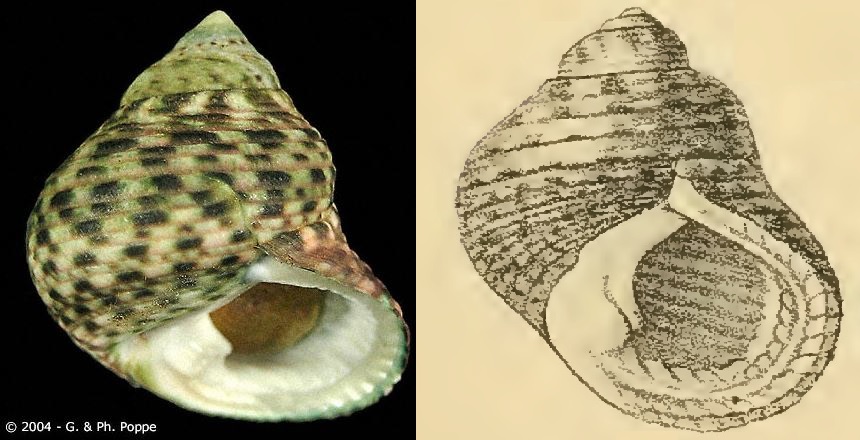
| Fig. 1. Monodonta labio at Labrador Park rocky shores. Photo: © 2014 Tan W. T. |
Table of Contents
 |
| Fig. 2. Monodonta labio sighted in Pulau Hantu, Singapore. Photo: © Ria Tan. Licensed under Creative Commons Attribution-NonCommercial-NoDerivs 2.0 Generic license. |
1. Nomenclature
Scientific Name: Monodonta labio (Linnaeus, 1758)
Original Combination: Trochus labio Linnaeus, 1758
Synonyms:
- Trochus novaezeelandica Röding, P.F., 1798
- Monodonta immanis Fischer, 1880
- Monodonta labio granulata Pilsbry, H.A., 1890
- Monodonta melaurchelonis Philippi
- Monodonta tuberculata A. Adams, 1853
Common Name: Toothed Top Shell Snail, or Lipped Periwinkle
2. Description
(i) Original Description
The genus Monodonta was first separated from Linnaen genus Trochus by Lamark in 1801, T. labio being given as the type [17]:
Genus Monodonta Lamarck, 1799
"Shell imperforate, turbinate, ovate or globose-depressed, the periphery rounded; surface smoothed or spirally ridged; columella simple, arcuate or spread upon the base at its insertion; below tuberculate, swollen, ending in a tooth or simple; outer lip smooth or lirate within" [17]
(ii) Shell
[15, 16] For more details on the gastropod shell, click here.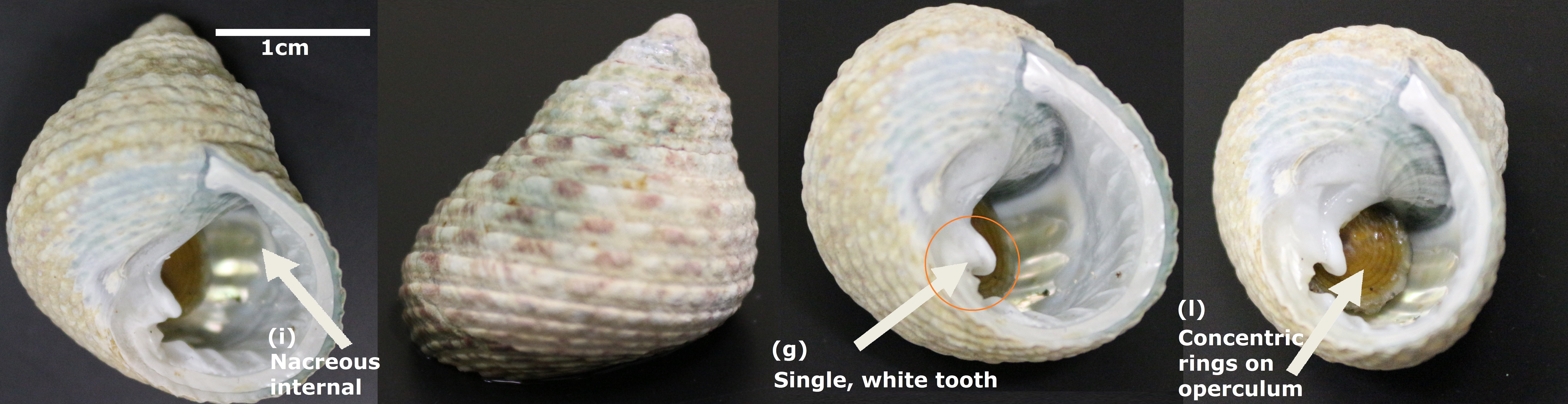 |
| Fig. 3. Monodonta labio (after preservation in 70% ethanol), with some key features labeled. Photos: © 2014 Tan W. T. |
(a) Length of shell varies from 1.5 cm to 4.5 cm
(b) Shell conical to globose in shape, asymmetrical, usually with a flattened base
(c) Body whorl is swollen and the penultimate whorl somewhat less.
(d) Shell displays a rounded keel
(e) Shell thick and coarse with rough, grained surface having spirals of rounded bumps
(f) Outside of shell extremely variable in colour, often grey or greenish grey
(g) Columella has a single prominent, blunt tooth (Mono donta means 'one-toothed'); Single, large tooth-shaped structure at the shell opening is white and smooth
(h) Inner edge of the outer lip shows a number of smaller knobs
(i) Shell internally nacreous
(j) Umbilicus present
(k) Aperture usually rounded, siphonal canal lacking
(l) Operculum thin and of a horn-like material, with multi-spiral concentric rings
(iii) Animal
[15, 16](a) Body pale
(b) Large foot pale on the underside, mottled greenish on the upper side
(c) Edge of mantle fringed with long tentacles
(d) A pair of long tentacles at the head
3. Biology
(i) Habitat
M. labio occurs in a wide range of intertidal habitats, including rocky, cobble and boulder shores as well as mangroves, but is more commonly found on sheltered or semi-exposed rocky shores [1, 7].
 |
| Fig. 6 and 7. Rocky shores in Sentosa (left) and Labrador Park where Monodonta labio is known to be found. Photos: © 2014 Tan W. T. |
These snails can also be found on artificial substrates such as sea walls [9].
 |
| Fig. 8 and 9. Seawalls in Pulau Hantu (left) and Labrador Park where Monodonta labio is known to be found. Photos: © 2014 Tan W. T. |
(ii) Feeding
M. labio is a herbivorous snail that forages on the surfaces of rocks and boulders to feed on epilithic microalgae [13]. When inactive, it can be found resting in less conspicuous sites such as in rock crevices or under boulders. |
|
| Fig. 10. Monodonta labio grazing over rocks in Labrador Park. Photo: © 2014 Tan W. T. |
Video showing mouth and foot of Monodonta labio during feeding. Photo: © 2013 Tsunokurage Permission granted |
(iii) Behaviour
Vertical Migration
In a study by Tanaka (1996a), M. labio was found to migrate vertically on the intertidal zone throughout the year and the direction of migration varied with the season and size of the snails. Smaller snails exhibited upward migration in summer whilst larger snails progressively migrated downwards to the lower intertidal zone throughout the year, most notably during summer [13].
Upward migration of smaller M. Iabio was postulated to be a result of the seasonal fluctuation in algal abundance, which affects growth rates of the snails. Small snails were found to migrate upwards from the middle to upper intertidal zone in summer during which the algal abundance in the upper zone became similar to that in the middle zone. Thus, M. labio may select and migrate between intertidal zones in response to algal abundance [13].
On the other hand, the downward migration of larger M. labio was hypothesized to be a result of larger snails seeking refuge from the high temperature in summer. The heat coma temperature of M. labio is 39.6°C so the snails need to relieve temperature and desiccation pressures during emersion periods [2]. It is probable that the lack of adequate refuges such as rock crevices, undersides of boulders and rock pools drives the downward migration of larger M. labio in summer [13].
Trail Following
Like most gastropods, M. labio secretes a thin layer of mucus over the substratum and glides over it, leaving behind a mucus trail [7]. Certain species of gastropods exhibit conspecific trail-following as part of aggregation and mating behaviours, as well as enhancing discovery of food sources [4, 5].
In the case of M. labio, experimental manipulations suggest that M. labio do not utilize trail-following for orientation but rather, for energetic benefits through reducing production of their own mucus or ingesting existing mucus [7]. Thus, it is possible that M. labio reaps energy savings in locomotion through trail-following.
(iv) Reproduction
M. labio is known to be a dioecious and iteroparous broadcast spawner spawning multiple times per reproductive season [14]. Eggs are laid singly in sea water and fertilization occurs externally [12]. Mature females of M. labio release their eggs gradually and always contained some eggs at any time during the spawning season. Having multiple spawning events within a single reproductive season is believed to be a response to unpredictability in recruitment success [14].
It also appears that the reproductive season of M. labio decreases with latitude, varying from two month in Asamushi, northern Japan, to seven months in Kyushu, Southern Japan [14]. A similar observation was also reported in another monodontine species, Monodonta lineata, in Europe [10]. It is postulated that a higher rate of repopulation failure in the northern regions due to temperatures being too low for successful establishment after spawning contributes to this latitudinal trend [10].
4. Distribution
M. labio is an Indo-Pacific species of mollusc, occurring over a wide range of locations, including Central and East Indian Ocean, Indo-China, Indo-Malaysian Oceania, the Philippines, the Persian Gulf, West Indian Ocean to Micronesia, Western Pacific, Micronesia and Australia [3, 12].
| Fig. 11. Global distribution of Monodonta labio. Illustration: © 2013 The Global Biodiversity Information Facility. Licensed under Creative Commons Attribution-ShareAlike 3.0 license. |
Locally, M. labio can be found along many shores including natural rocky shores as well as artificial seawalls.
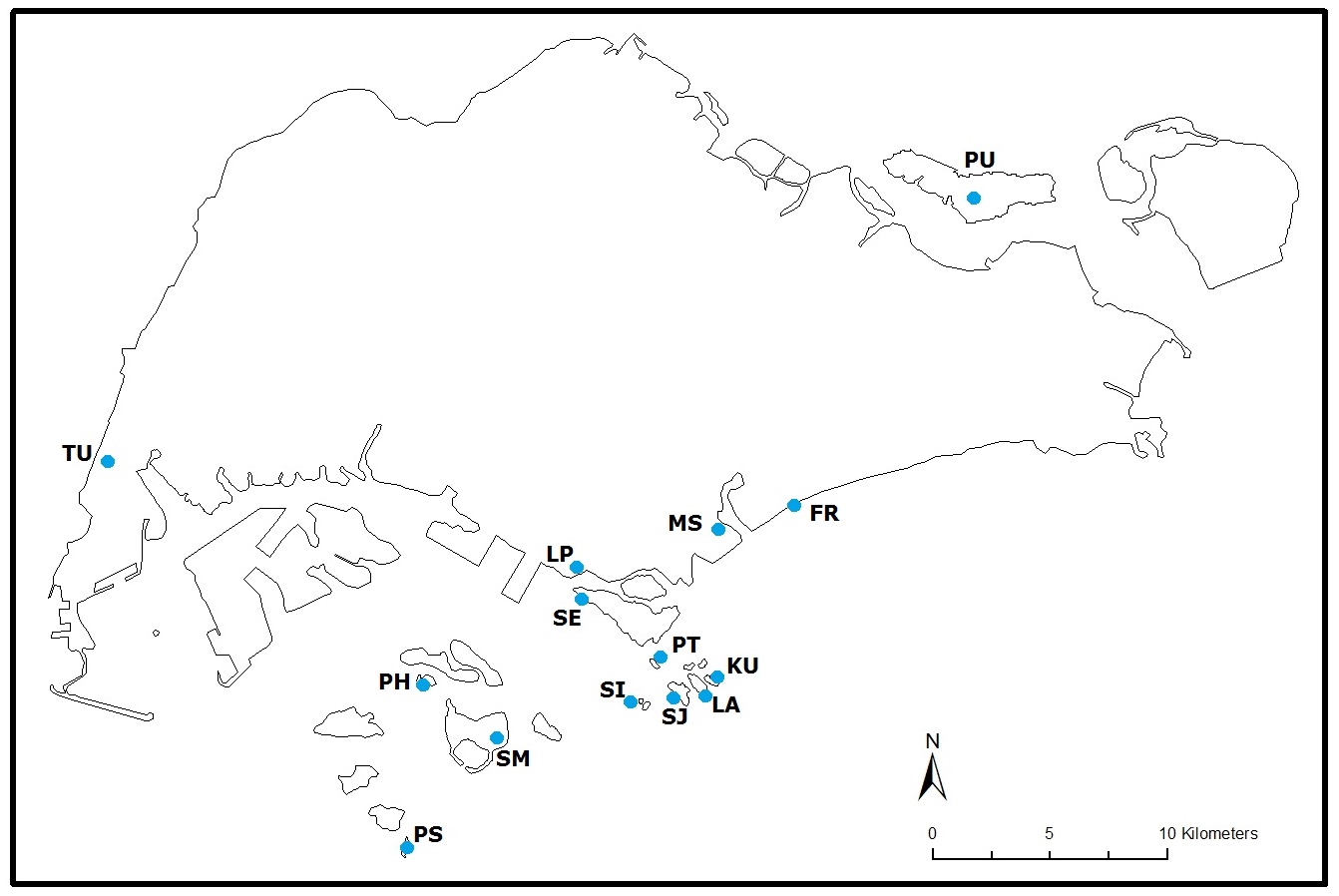 |
| Fig. 12. Distribution of Monodonta labio in Singapore based on wildsingapore.com and Lee et al., 2009. FR, Fort Road; KU, Kusu Island; LA, Lazarus Island; LP, Labrador Park; MS. Marina South; PH, Pulau Hantu; PT, Pular Tekukor; PU, Pulau Ubin; SE, Sentosa; SI, Sister's Island; SJ, St. John's Island; SM, Pulau Semakau; TU, Tuas. Illustration: © 2014 Tan W. T. |
5. Diagnosis
Keys are useful tools for the identification of molluscs. Below is a key provided by the Food and Agriculture Organization (1998) to aid in the identification of common mollusc species occurring in the Indo-Pacific region, such as the M. labio [12].
| Fig. 13. Key to identifying common molluscs in the Indo Pacific region. Key: © Poutiers, 1998. |
(i) Comparison between similar families: Trochidae vs. Turbinidae
Shells of the family Trochidae may sometimes be confused with those of the Turbinidae. Some distinguishing morphological traits between the two families commonly occurring in the Indo-Pacific region is provided below [12].
| Trochidae |
Turbinidae |
||||
|
|
||||
|
|
(ii) Comparison between similar species: M. labio, M. australis, M. confusa and M. nebulosa
Certain species of the genus Monodonta may look similar as well. Below is a table summarizing key diagnostic differences between M. labio and three other species of the genus Monodonta [17].
6. Classification
| Kingdom: |
Animalia |
| Phylum: |
Mollusca |
| Class: |
Gastropoda |
| Subclass: |
Vestigastropoda |
| Order: |
Archaeogastropoda |
| Superfamily: |
Trochoidea |
| Family: |
Trochidae |
| Subfamily: |
Monodontinae |
| Genus: |
Monodonta |
| Species: |
labio |
7. Phylogeny
In an endeavor to construct the phylogeny of gastropod molluscs using morphological characters, Ponder and Lindberg (1997) analysed 117 characters from 40 taxa, predominantly ‘prosobranchs’. Of the 117 characters included in the analyses, nine are shell characters, two opercular, two muscular, four ctenidial, 12 renopericardial, 24 reproductive, 27 of the digestive system, 32 of the nervous system and sense organs; the remainder are developmental (3) and of the foot and hypobranchial gland [11].
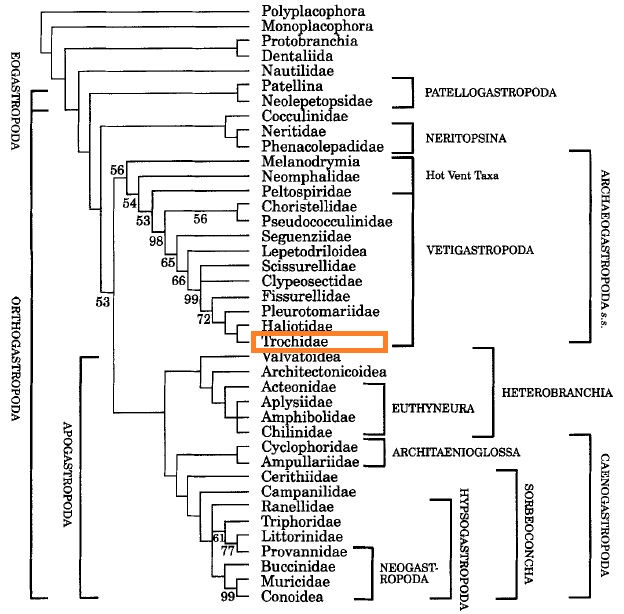 |
| Fig. 24. Phylogeny of gastropods constructed from morphological characters. Diagram: © Ponder and Lindberg, 1997. Permission pending. |
Gastropods appear to be a monophyletic clade, and divide into two primary groups, the Eogastropoda (incorporating the patellogastropods and their (probably sinistrally coiled) ancestors) and the Orthogastropoda- the remainder of the gastropods. Orthogastropoda comprises several well defined clades, one of which is the vetigastropod clade encompassing groups previously included in the paraphyletic Archaeogastropoda (fissurellids, trochoideans, scissureiloideans, halioroideans pleurotomarioideans) as well as lepetodriloidean and lepetelloidean limpets and seguenzids [11].
8. Molecular Phylogeny
Trochoidea is an extremely diverse superfamily of marine gastropods comprising of five families, namely Trochidae, Calliostomatidae, Turbinidae, Liotiidae and Solarielliidae. In particular, Trochidae is possibly the largest and most diverse trochoidean family with over 600 species distributed throughout the Pacific, Indian and Atlantic oceans [6, 18]. In a recent molecular study of the gastropod family Trochidae, a total of 110 trochid species were sampled and four genes (i.e. nuclear 28S rRNA gene, 12S rRNA, cytochrome oxidase subunit I and 16S rRNA) were analysed to reconstruct the Trochoidea family systematics [18].
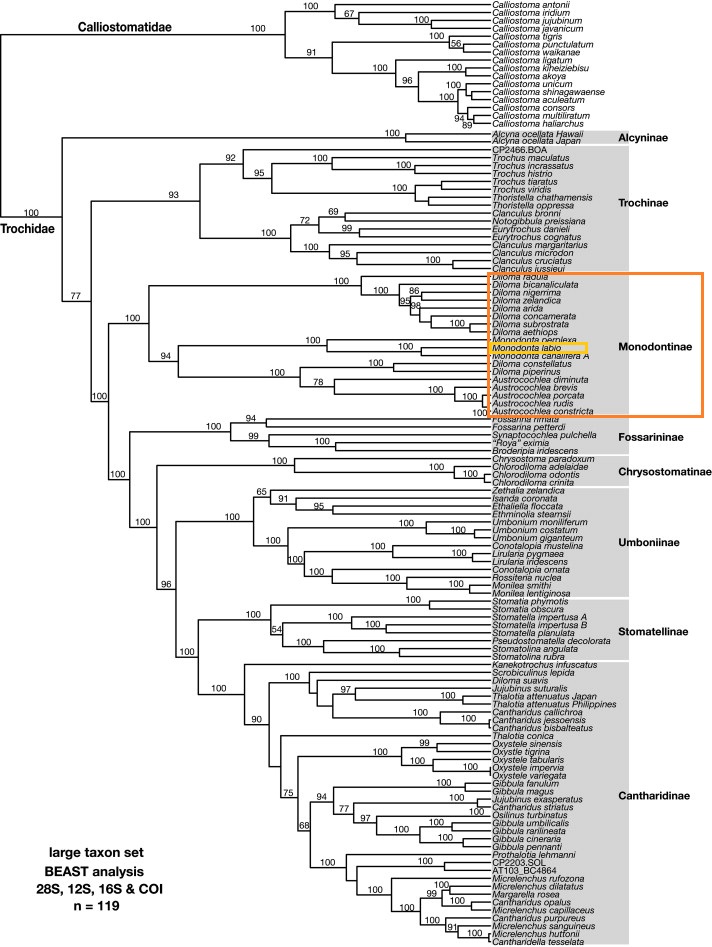 |
| Fig. 25. Molecular phylogeny for Trochidae and Calliostomatidae based on analysis of concatenated sequences from four genes (28S rRNA, 12S rRNA, 16S rRNA and COI) produced by Bayesian analysis incorporating an uncorrelated relaxed, lognormal clock using BEAST. Diagram: © Williams et al., 2010. Permission pending. |
Subfamily Monodontinae is shown to be monophyletic and redefined to consist of only three genera: the nominotypical genus Monodonta Lamarck, 1799 (four species), Diloma Philippi, 1845 (11 species) and Austrocochlea Fischer, 1885 (five species). The conventionally monodontine genera Oxystele Philippi, 1847 and Osilinus Philippi, 1847 are being shifted to subfamily Cantharidinae [18].
Another study on subfamily Monodontinae revealed new insights to the systematics of Monodonta labio which were not reported from morphological assays [3]. Analysis of two mitochondrial genes (16S and cytochrome oxidase 1, COI) and one nuclear gene (actin) extracted from the foot tissue of 30 species of monodontine topshells (family Trochidae) produced molecular data to construct well-supported phylogenetic trees (based on DNA sequences) [3].
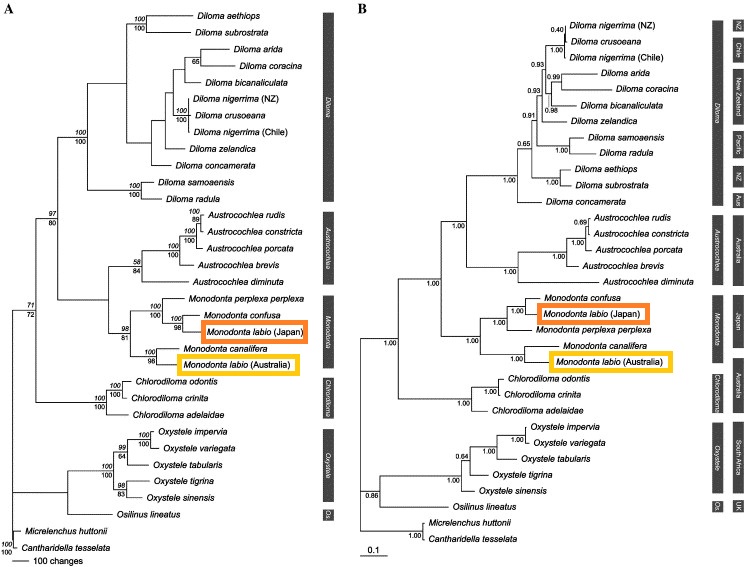 |
| Fig. 26. A (left): Weighted maximum parsimony phylogram generated from combined 16S, COI and actin sequence data. B: Bayesian phylogram generated from combined 16S, COI and actin sequence data. Diagram: © Donald et al., 2005. Permission granted. |
At the species level, within Monodonta, the two Japanese taxa commonly referred to as M. labio labio and M. labio confusa are revealed to be genetically divergent. Thus, they are treated as separate species, M. labio and M. confusa respectively, as indicated in the phylograms. In addition, M. labio individuals acquired from Australia turned out to be genetically distinct from the Japanese M. labio, suggesting that the Indo-Pacific taxon M. labio may be a species complex harbouring cryptic species [3].
Hence, molecular data may reveal cryptic species in species complexes which would otherwise be difficult to elucidate based on morphology alone. Molecular data may thereby aid morphology in constructing and re-examining systematics of species.
9. Commercial Value
Larger or more common species of Trochidae are a food source for coastal communities in parts of Southeast Asia and Southwest Pacific [12]. M. labio is known to be harvested for food in some regions such as in Amakusa, Japan. It is thought that the harvesting pressure of large M. labio by humans contributes to a smaller maximum size in Amakusa as compared to other regions in Japan such as Shima Peninsula and Kominato where M. labio is not eaten [8].
As the aperture of M. labio is nacreous inside, the shells may be exploited for shellcraft [12]. The entire shells are also gathered from various coasts around the world and sold to shell collectors.
| Fig. 27. Screenshot of ebay site, with Monodonta labio shells for sale. |
 |
| Fig. 28. Screenshot of vintage-toys-for-sale.com site, depicting sale of Monodonta labio. |
10. Glossary
Click on the term again to return to its original location within the main textAperture: the opening of the shell
Columella: the "little column" at the axis of revolution of the shell, often only visible when the shell is broken, or viewed as an X-ray image
Columellar tooth: a raised projection on the inner lip of a columella in the direction of the aperture
Cryptic species: a group of organisms that are typically very closely related but their precise classification and relationships cannot be easily determined
Dioecious: a characteristic of a species, meaning that it has distinct male and female individual organisms
Epilithic: growing on the surface of rocks
Globose: height and the width of the shell are approximately the same
Iteroparous: reproduces multiple times over the course of its lifetime
Keel: a spiral ridge marking a change in slope
Lip: margin of the aperture
Nacreous: iridescent, mother of pearl layer found on the inner shell of the mollusc
Operculum: "trapdoor" to close the aperture of the shell
Siphonal canal: an extension of the aperture in certain gastropods
Umbilicus: deep depression on the under s towards the spire; found in shells where the whorls move apart as they grow
Whorl:each one of the complete rotations of the shell spiral
References
[1] Chin, I. (2003) Variation in Monodonta labio among different intertidal habitats in Hong Kong. PhD Thesis, The University of Hong Kong, Hong Kong.
[2] Cleland, J. D. and McMahon, R. F. (1988) Upper thermal limit of nine intertidal gastropod species from a Hong Kong rocky shore in relation to vertical distribution and desiccation associated with evaporative cooling. In: Morton, B. (ed) Proceedings of the Second International Marine Biological Workshop: the marine flora and fauna of Hong Kong and southern China, Hong Kong, 1986. Hong Kong University Press, Hong Kong, p 1141–1152.
[3] Donald, K. M., Kennedy, M. and Spencer, H. G. (2005) The phylogeny and taxonomy of austral monodontine topshells (Mollusca: Gastropoda: Trochidae), inferred from DNA sequences. Molecular Phylogenetics and Evolution, 37: 474–483.
[4] Erlandsson, J. and Kostylev, V. (1995) Trail-following, speed and fratal dimension of movement in a marine prosobranch, Littorina littorea, during a mating and non-mating season. Marine Biology, 122: 87–94.
[5] Hawkins, S. J. and Hartnoll, R. G. (1983) Grazing of intertidal algae by marine invertebrates. Oceanography and Marine Biology: an Annual Review, 21: 195–282.
[6] Hickman, C. S. and McLean, J. H. (1990) Systematic revision and suprageneric classification of trochacean gastropods. Natural History Museum of Los Angeles County Science Series, 35: 1–169.
[7] Hutchinson, N., Davies, M. S., Ng, J. S. S. and Williams, G. A. (2007) Trail following behaviour in relation to pedal mucus production in the intertidal gastropod Monodonta labio (Linnaeus). Journal of Experimental Marine Biology and Ecology, 349: 313–322.
[8] Iijima, A. (2001) Growth of the intertidal snail, Monodonta labio (Gastropoda, Prosobranchia) on the Pacific coast of Central Japan. Bulletin of Marine Science, 68 (1): 27–36.
[9] Lee, A. C., Tan, K. S. and Sin, T. M. (2009) Intertidal Assemblages on coastal defence structures in Singapore I: A Faunal study. Raffles Bulletin of Zoology, 22: 237–254.
[10] Lewis, J. R. (1986) Latitudinal trends in reproduction, recruitment and population characteristics of some rocky littoral molluscs and drripedes. Hydrobiologia, 142: 1–13.
[11] Ponder, W. F. and Lindberg, D. R. (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zoological Journal of the Linnean Society, 119: 83–265.
[12] Poutiers, J.M. (1998) Gastropods. In: Carpenter, K. E. and Niem, V.H. (eds) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 1. Seaweeds, corals, bivalves and gastropods. Rome, FAO. p 1–686.
[13] Takada, Y. (1996a) Vertical migration during the life history of the intertidal gastropod Monodonta labio on a boulder shore. Marine Ecology Progress Series, 130: 117–123.
[14] Takada, Y. (1996b) Vertical variation in fecundity of the intertidal gastropod Monodonta labio caused by different growth rates between tidal zones. Ecological Research, 11: 371–379.
[15] Tan,L. W. H. and Ng, P. K. L. (1988) A Guide to Seashore Life. The Singapore Science Centre, Singapore.
[16] Tan, R. (2008) Toothed top shell snail Monodonta labio. http://www.wildsingapore.com/wildfacts/mollusca/gastropoda/trochidae/labio.htm (Accesses 29 September2014).
[17] Tryon, G. W. (1889) Manual of Conchology; Structural and Systematic, with illustrations of the speices. Volume XI: Trochidae, Stomatidae, Pleurotomariidae, Halitidae. Academy of Natural Sciences, Philadelphia.
[18] Williams, S. T., Donal, K. M., Spencer, H. G. and Nakano, T. (2010) Molecular systematics of the marine gastropod families Trochidae and Calliostomatidae (Mollusca: Superfamily Trochoidea). Molecular Phylogenetics and Evolution, 54 (3): 783–809.